
Ask anyone in pharma what keeps them up at night, most of them will say ‘Compliance’ is near the top of the list. It’s always been important, but now it’s layered with faster approvals, stricter audits, and a growing web of global regulations that don’t leave much room for error.
However, while the regulations have evolved, the day-to-day reality inside most organizations hasn’t shifted nearly as fast. This growing gap between regulatory expectations and day-to-day operations is creating many challenges that teams face today.
In this blog, we’ll uncover the top six compliance pain points pharma teams grapple with daily and how Techwave’s tailored SAP solutions turn those vulnerabilities into strengths, embedding compliance right into the DNA of your operations.
Top 6 weak spots in the pharmaceutical industry where compliance can break down

- Regulatory pressure is rising: Authorities like the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) are stepping up inspections with less lead time and more scrutiny.
Updates to the Code of Federal Regulations (21 CFR Part 11), EU Annex 1, and Good Automated Manufacturing Practice guidelines (GAMP for manufacturers and users of automated systems in the
pharmaceutical industry) have made it clear that documentation alone is not enough. Companies must show that systems are in control and processes are consistently followed. - Data is everywhere and nowhere at once: Information lives across quality systems, lab software, batch records, and enterprise platforms. But when those systems aren’t aligned, teams end up piecing together reports manually.
This slows response times and makes it harder to protect data integrity, which remains a critical focus under ALCOA+ principles (a set of principles used to ensure data integrity in regulated industries like pharmaceuticals). - Supply chain visibility falls short: Late deliveries, incomplete documentation, and limited supplier accountability continue to impact product release timelines.
Serialization requirements under the Drug Supply Chain Security Act (DSCSA) and the EU Falsified Medicines Directive (EU FMD) are non-negotiable, but many organizations still lack complete oversight of materials in motion.
- R&D is scaling faster than internal systems: As research portfolios grow, so does the pressure on internal processes. Teams managing clinical programs, budgets, and submissions often work across disconnected systems. Without reliable coordination, timelines slip and regulatory expectations become harder to meet.
- Cybersecurity risks are tied to compliance outcomes: Clinical data, electronic batch records, and intellectual property require secure, validated environments. A data breach or access control failure can quickly escalate into regulatory action, especially when systems support GxP operations.
Good “x” Practice encompasses various regulations and guidelines governing different disciplines (“x”) of pharmaceutical operations like manufacturing (GMP),
clinical trials (GCP), laboratory work (GLP), or distribution (GDP). - External partners are part of the process but outside the system: Contract Manufacturing Organizations (CMOs), Contract Research Organizations (CROs), and other vendors handle critical operations. But if their systems are not aligned with internal ones, visibility suffers, and risks go unnoticed until an inspection brings them to light.
Building compliance into the core of pharma and life sciences
The question is – how can pharma and life sciences companies embed compliance into the core of their operations and keep lawsuits, penalties, and stress at bay while saving cost?
What they truly need is an ERP system that brings every critical function, including quality, production, finance, inventory, and compliance, into one connected, reliable environment.
And what could be better than SAP S/4HANA ? It’s built to support the unique demands of regulated industries, with real-time visibility, traceability, and process control that help teams stay audit-ready without slowing down operations.
As an SAP Gold Partner, Techwave works closely with pharma and life sciences companies to deliver end-to-end SAP implementation, from blueprint to validation and post-go-live support.
Here is how we help our clients align SAP to solve the very challenges discussed above.

- Digitized quality management built for GxP and audit readiness
Techwave configures SAP Quality Management (QM) to digitize inspections, manage deviations, and automate Corrective and Preventive Actions (CAPAs).
Each quality record links directly to batch data, materials, and equipment. Documentation aligns with ALCOA+ principles and GxP expectations, helping teams stay prepared for FDA audits and regulatory inspections. - Unified data model that supports real-time traceability
With SAP S/4HANA , we centralize production, quality, inventory, and finance in a single platform. This setup eliminates silos and provides real-time access to accurate data, enabling faster reporting and reducing compliance risks across the enterprise. - Serialization and supply chain visibility to meet global regulations
We implement SAP Advanced Track and Trace for Pharmaceuticals (ATTP) and SAP Global Batch Traceability (GBT) to enable unit-level product tracking.
These tools help meet the Drug Supply Chain Security Act (DSCSA) and European Union Falsified Medicines Directive (EU FMD) requirements while giving teams complete visibility across packaging, distribution, and storage. - R&D and CAPEX project oversight with full control
We give teams visibility into timelines, budgets, and regulatory deliverables through SAP Project System (PS) and Investment Management (IM). This helps coordinate cross-functional work and ensures accountability across clinical development, tech transfers, and infrastructure investments. - Compliance-driven access, security, and system validation
Using SAP Governance, Risk, and Compliance (GRC), we configure electronic signatures, audit trails, and access controls to meet 21 CFR Part 11 and GAMP 5 requirements. This safeguards validated systems while supporting traceability and accountability across critical processes. - Integrated oversight of external partners and vendors
By extending workflows within SAP Supplier Lifecycle Management (SLM), we help companies manage vendor qualification, performance, and compliance. External partners like CMOs and CROs operate within the same standards, reducing risks during audits and inspections.
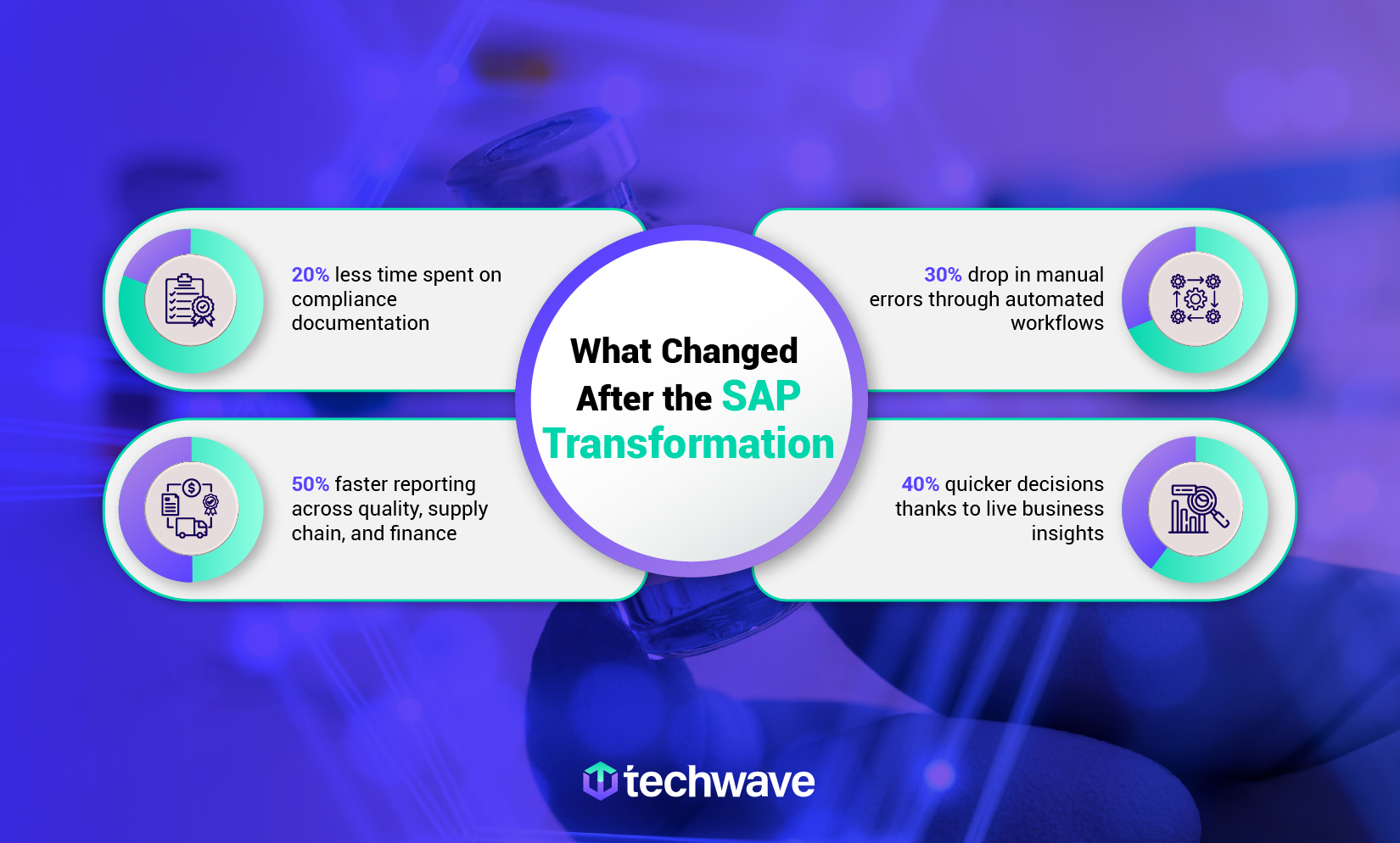
How Techwave helped a global API leader streamline compliance and scale smarter
A global manufacturer producing over 70 high-value Active Pharmaceutical Ingredients (APIs) for some of the biggest names in pharma and biotech was hitting a wall. Their systems couldn’t keep pace with growing regulatory pressure, scattered data made decision-making slow, and compliance processes were dragging teams down instead of lifting them up.
We came on board as their SAP transformation partner , helping them move to a smarter, more connected foundation with SAP S/4HANA . From clearing up supply chain blind spots to simplifying plant safety tracking, we helped build a system that fits how their business actually works across teams, borders, and audits.
If you’re facing similar challenges and want to see what’s possible with the right SAP setup, get in touch with us. We’d be happy to explore how we can help.
